Collaboration with multiple stakeholders to launch innovative biopharmaceuticals in Japan
Drug discovery and development is a good example of the type of knowledge-intensive industry that Japan is striving to cultivate. Because the market for drug development is growing as the population ages, this industry is positioned as a next-generation growth industry in the government’s Japan Revitalization Strategy. In today’s drug industry, it has become difficult to rely solely on low-molecular-weight drugs produced through the conventional method of chemical synthesis, and we must turn to biopharmaceuticals made using biotechnology. Japan, however, has been noted as being slow to venture into biopharmaceutical development compared to Western countries, and for this reason, the Ministry of Education, Culture, Sports, Science and Technology initiated a five-year "Basic Science and Platform Technology Program for Innovative Biological Medicine" in 2014. To strengthen Japan’s ability to compete in the international market, we must solve technological problems faced in this field and out-license the resulting intellectual property and knowledge to companies so that they can develop innovative next-generation biopharmaceuticals.
Japan has a high level of fundamental research, and in drug development, there have been many successes in basic research and a wealth of information acquired about potential drug targets. Various policies implemented in recent years aimed at accelerating the practical application of research have laid the groundwork for taking basic research at universities—mainly findings regarding low-molecular-weight drugs—from the drug development process through investigator-initiated clinical trials to evaluate effectiveness in humans in order to establish proof of concept (PoC). It is now possible for academic researchers, through close collaboration with contract research organizations and contract manufacturing organizations (CROs/CMOs), to be involved in the development process of a first-in-human class drug, from hit compound discovery through structural optimization, GMP synthesis and formulation, and a panel of pre-clinical GLP pharmaco-toxicological studies all the way through investigator-initiated clinical trials. Low-molecular-weight drugs can be manufactured with uniform structure and quality because they are created through chemical synthesis. Furthermore, their structure and quality are easily analyzed with the technology we currently have. This explains why academic researchers have had success in drug development and building expertise in this area with relative ease.
Biopharmaceuticals, in contrast, have a high molecular weight and much more complex structures. Parameters, such as conditions for culturing, purification, and concentration must be strictly controlled to ensure a certain standard of quality, making it difficult to ensure the equivalency of large quantities of products. (This is also clear from the difficulty of developing biosimilar generic versions of biopharmaceuticals compared with developing generic versions of low-molecular-weight drugs.) There is very little collaboration between academic researchers and CROs/CMOs that specialize in biopharmaceuticals (bio CROs), so that even if academic researchers make theoretically successful proposals for innovative biopharmaceuticals, there are major challenges in bringing those drugs to clinical trials and eventually market. Furthermore, unlike with low-molecular-weight drugs, many pharmaceutical companies have not built up sufficient experience or expertise regarding biopharmaceuticals. Biopharmaceuticals, particularly therapeutic antibodies, account for over one-third of staple products in the global pharmaceutical market. However, only a few pharmaceutical companies have brought new drugs developed in Japan to market. As this illustrates, academic researchers, bio CROs, and pharmaceutical companies (bio ventures) are building experience in this field independently of each other, so promoting ties and cooperation among these groups is an important next step.
If Japan is to create innovative next-generation biopharmaceuticals, we must do more than simply discover elemental technology, candidate drugs or new concepts; we must also identify and resolve a multitude of issues. For example, we should increase added value and improve the likelihood that candidate drugs will actually be produced by establish a forum where academic researchers, bio CROs, and bio ventures can being together their experience and expertise. Unless these steps are taken, regardless of whether Japanese universities make academic breakthroughs—which I think they are capable of doing—issues with manufacturing technology, intellectual property, pharmaceutical regulations, and communication networks will prevent us from creating innovative next-generation biopharmaceuticals.
Many promising candidate drugs discovered by academic researchers are in the the cultivation phase of development. It is at these early stages that we must provide either strong motivation or support for an exit strategy of out-licensing or commercialization. Furthermore, because pharmaceutical companies cannot license every drug candidate introduced by academic researchers, universities must cultivate those that cannot immediately be out-licensed through material optimization, quality assurance, and GLP studies in accordance with the Pharmaceutical Affairs Law, collaborating with bio CROs as necessary . Through such a process, researchers and other involved parties will develop the experience and expertise needed for low-molecular-weight drug development. Although this slightly diverges from the purpose of this program, I believe that along with tackling various challenges in research and development, it is important to discuss and implement measures to solve these kinds of issues.
Because biopharmaceuticals have a brief history compared to low-molecular-weight drugs, there remain various issues in their development. There also is, however, untapped potential for innovative drugs to be born in this field. In fiscal 2014 and 2015, we selected 26 new research and development projects in a variety of areas. All of these projects involve drug candidates or elemental technologies that have the potential of producing innovative biopharmaceuticals, so I am looking forward to seeing progress in these research projects. We are fortunate to have experts with vast experience in biopharmaceutical development join us to determine how the program is implemented. I hope we can all work together to manage the program in a flexible and productive manner.
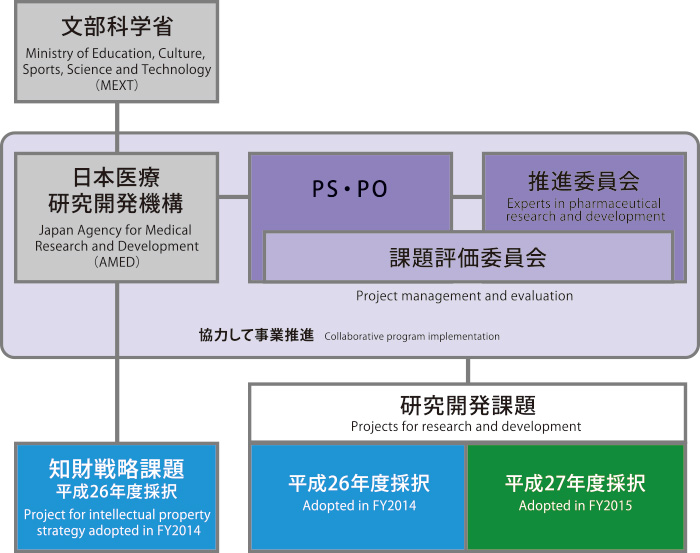
In April 2015, the program was transferred to the auspices of the Japan Agency for Medical Research and Development (AMED) and became a pillar of the "Project for Drug Discovery and Development" strategy. We also hope to link it with other programs planned by AMED and to maintain open dialogue with regulatory authorities and private businesses such as bio ventures, bio CROs, and pharmaceutical companies. I sincerely request your support in accomplishing our goals.