Research and Development Projects Adopted in FY2014
Structure-based innovation of genome-editing tool (CRISPR) system and its medical application
Project Leader:Nureki Osamu
Professor, Graduate School of Science, The University of Tokyo

Our laboratory promotes 3 projects, that is, 1.
Membrane transport mechanism by channels and transporters, 2.
Mechanism of gene expression regulation by non-coding RNAs, and 3.
Mechanism of disease pathogenesis by chronic inflammation.
To elucidate molecular mechanisms of these biological processes, we first determine the three-dimensional structures of protein and nucleic acid molecules essential for these biological processes, and verify the mechanisms by complementary in vitro and in vivo functional analyses designed on the basis of the structures.
In this "Creative drug-generating base technology development project", we focus on CRISPR-Cas9, acting in DNA double strand break depending on guide RNA, from the molecular structure level to medical application.
While CRISPR-Cas9 has brought innovation to genome editing field and has already been used for medical studies, the system has still several problem for application to practical medical treatment.
For example, the present CRISPR-Cas9 system requires to recognize PAM sequence on the genome, a 2-5 nucleotide sequence just downstream the cleavage site.
To resolve the restriction, we will first solve the crystal structures of quaternary complexes of various Cas9 proteins recognizing different PAM sequences to fully understand the specific recognition mechanism of PAM sequence by Cas9, and will develop the Cas9 mutant set that can recognize any PAM sequence and cleave any genome sequence (The University of Tokyo Team).
Then, towards medical application, we will introduce the complex of recombinant Cas9 mutants and guide RNAs into semi-intact cells to establish evaluation system for simple, safe and generally-usable Cas9 system set: in particular, we will evaluate probability of homologous recombination and off-target by the use of a next-generation DNA sequencer.
Furthermore, using the semi-intact cell technology, we will develop the methodology of simultaneous visualization of a number of gene loci to quantitatively evaluate the off-target problem, one of the agenda for the present CRISPR-Cas9 (The University of Tokyo Team).
We have already established in vivo evaluation system of gene knock-out by genome editing using fertilized mouse ovum, and we will use the system to optimize the newly-developed improved CRISPR-Cas9 system (The Gunma University Team).
Furthermore, based on our recent study on DNA homologous recombination initiation mechanism, we will regulate endogenous homologous recombination efficiency in hematopoietic stem cells etc. using semi-intact cells to develop a new technology to overcome the low efficiency of in vivo homologous recombination, which is another agenda for the present CRISPR-Cas9 (The University of Tokyo Team).
Finally, combined all together, we will establish the methodology of treatment of pig model of human genetic diseases (X-SCID etc.) by transplantation of hematopoietic stem cells genome-edited by the new CRISPR-Cas9 set system (The Jichi Medical University Team).
<Figure1>
The crystal structure of the light-gated cation channel, channel rhodopsin, the optogenetics tool which has become the breakthrough of the neuroscience.
In our laboratory, we elucidated the structure and molecular mechanism first over the world.
<Figure2>
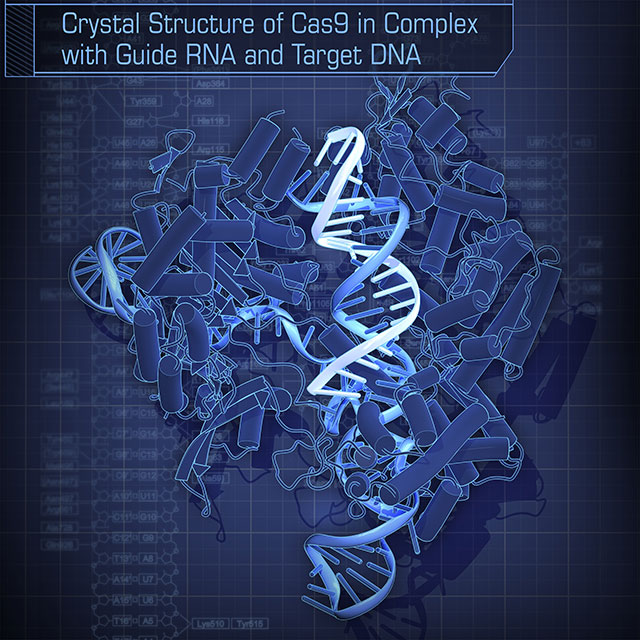
Cas9 is a next-generation genome editing tool. We solved the first crystal structure of Cas9 complexed with guide RNA and target DNA.
<Figure3>
Biomolecular crystallization.
<Figure4>
X-ray crystallographic analysis.