News Releases & Research Results Development of Precision Medicine for Gastrointestinal Cancers: Utility of Liquid Biopsy in Genomic Analysis
News Releases & Research Results
National Cancer Center Japan
Japan Agency for Medical Research and Development
Key points
- This study demonstrates for the first time in the world that employing a blood test to detect genomic alterations (liquid biopsy) in screening of patients with gastrointestinal cancer shortens the turn-around time to return the test results and accelerates enrollment of patients to clinical trials, compared to conventional tissue biopsy.
- This is the first large-scale study that reports a comparison of the utility between tumor tissue biopsy and liquid biopsy for screening patients in clinical trials.
- Application of liquid biopsy as screening test*1 to many clinical trials is expected to broaden opportunities for more patients to receive the best medical care.
Outline
National Cancer Center (President: Hitoshi Nakagama, Tokyo, Japan) and Hospital East (Director: Atsushi Ohtsu, Chiba, Japan) demonstrated for the first time in the world that introducing a blood test to detect genomic alterations in patients with gastrointestinal cancer into screening for clinical trials achieves a shorter turn-around time to return the results, and expands patient enrollment in trials.
Liquid biopsy enables repeated testing of blood samples, eliminating the need for collecting tumor tissues. While conventional tissue collection is significantly invasive and sometimes leads to delays of treatment decisions, liquid biopsy would allow more patients to reach optimal therapeutic drugs with reduced burden to their bodies given its utility demonstrated in this study.
In this study, the turn-around time for receiving genomic analysis results, the enrollment rate in, and the drug efficacy in clinical trials were compared between the genomic analysis results from “GI-SCREEN-JAPAN (tumor tissue biopsy)”, which targets advanced gastrointestinal cancers, and those from “GOZILA study (liquid biopsy)”. The study revealed that liquid biopsy shortens turn-around time by approximately 22 days till obtaining results and raises patient enrollment to eligible clinical trials based on the genomic analysis results as compared with tissue biopsy.
This study was conducted by Dr. Takayuki Yoshino, Head of Gastrointestinal Oncology Group, Dr. Yoshiaki Nakamura, Translational Research Management Division, Clinical Research Support Office, and their colleagues as part of GI-SCREEN-JAPAN and GOZILA Study, and the study results were published online (October 5, 2020) in an American scientific journal “Nature Medicine”.
Background
A large number of clinical trials which utilize information about genomic alterations are conducted all over the world to establish cancer precision medicine*2. In these, tumor tissue biopsies have been used for identification of genomic alterations associated with the patient’s cancer characteristics. However, unavailability of tumor tissue in some patients and the relatively long timeframe requirement for analysis of tissue biopsies have impeded the acceleration of such trials.
On the other hand, liquid biopsy technologies have evolved rapidly in recent years. It has been suggested that liquid biopsy may resolve issues associated with conventional tumor tissue biopsy as liquid biopsy allows genomic profiling without tumor tissue samples, and also as the turn-around time to return test results is shorter. However, there had been no large-scale studies to date that compared the utility of tissue and liquid biopsies.
Given this situation, a large-scale comparative study was conducted by National Cancer Center Hospital East by utilizing the platform of a nation-wide industry-academia collaboration project “SCRUM-Japan” *3.
Research methods and achievements
National Cancer Center launched a nation-wide industry-academia collaboration project “SCRUM-Japan” and has conducted “GI-SCREEN-JAPAN (currently MONSTAR-SCREEN)” since February 2014. GI-SCREEN-Japan is a nation-wide cancer genome screening project wherein NCC cooperates with major cancer specialty hospitals and university hospitals in Japan to deliver therapeutic drugs to advanced gastrointestinal cancer patients. In January 2018, NCC launched GOZILA study, a screening project in collaboration with Guardant Health Inc. (USA), in which blood samples of patients with advanced gastrointestinal tumors are analyzed by liquid biopsy (Guardant360® Assay) utilizing the platform of GI-SCREEN-Japan.
In this study, we compared 5743 patients enrolled in GI-SCREEN-Japan (tumor tissue biopsy) from February 2015 to April 2019 (4 years and 2 months) and 1787 patients enrolled in GOZILA Study (liquid biopsy) from January 2018 to August 2019 (1 year and 7 months) (Figure 1).
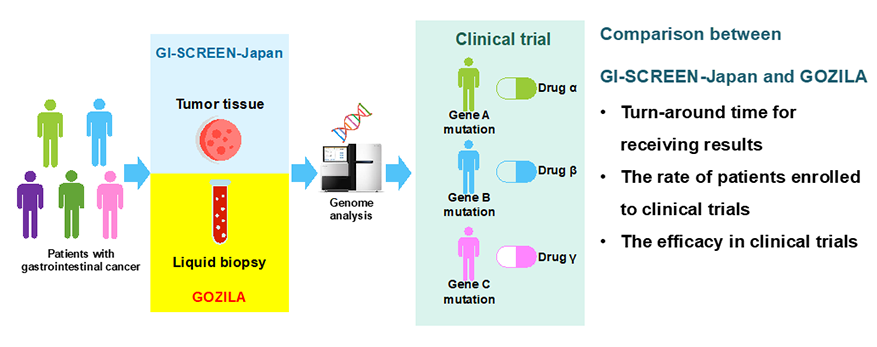
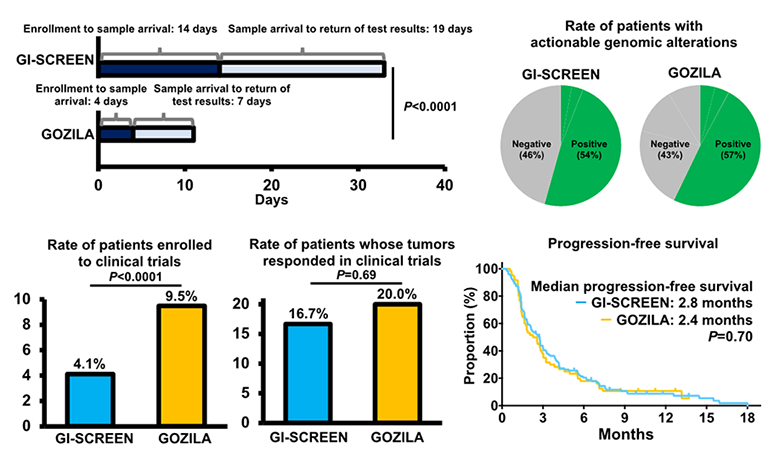
Comparison results (GI-SCREEN-Japan vs. GOZILA Study) (Figure 2)
- Project enrollment to sample arrival (median: 14 days vs 4 days, P<0.0001)
- Sample arrival to return of test results to patients (median: 19 days vs. 7 days, P<0.0001)
- Patients with actionable genomic alterations identified (54% vs. 57%)
- Patients enrolled in clinical trials of drugs matching detected genomic alterations (4.1% vs. 9.5%、P<0.0001)
- Patients whose tumors responded in clinical trials (16.7% vs. 20.0%, P=0.69)
- Progression-free survival in clinical trials (median: 2.8 months vs. 2.4 months, P=0.70)
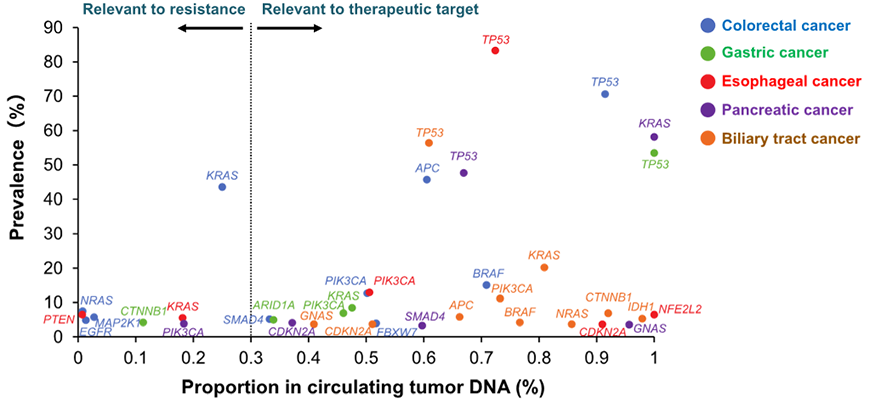
In addition, genomic profiling*4 by liquid biopsy in GOZILA study revealed useful biomarkers and several novel driver gene*5 mutations including NFE2L2 mutation in esophageal squamous cell carcinoma, GNAS mutation in pancreatic cancer and CTNNB1 mutation in biliary tract cancer, potential candidates of therapeutic targets.
the Future
Based on the results of this study, utilizing liquid biopsy as screening test in clinical trials is expected to deliver optimal treatments to many patients. In addition, the discovery of new driver gene mutations will potentially instigate clinical development of drugs for them. Indeed, several investigator-initiated trials have already been launched based on liquid biopsy results in GOZILA study. National Cancer Center Hospital East will continue to further establish genomic medicine using liquid biopsy to deliver optimal therapies to as many patients as possible.
Research Paper
- Journal name
- Nature Medicine
- Title
- Clinical Utility of Circulating Tumor DNA Sequencing in Advanced Gastrointestinal Cancer: SCRUM-Japan GI-SCREEN and GOZILA Studies
- Authors
- Yoshiaki Nakamura1,2,33, Hiroya Taniguchi1,2,33, Masafumi Ikeda3, Hideaki Bando4, Ken Kato5,6, Chigusa Morizane7, Taito Esaki8, Yoshito Komatsu9, Yasuyuki Kawamoto9, Naoki Takahashi10, Makoto Ueno11, Yoshinori Kagawa12, Tomohiro Nishina13, Takeshi Kato14, Yoshiyuki Yamamoto15, Junji Furuse16, Tadamichi Denda17, Hisato Kawakami18, Eiji Oki19, Takako Nakajima20, Naohiro Nishida21, Kensei Yamaguchi22, Hisateru Yasui23, Masahiro Goto24, Nobuhisa Matsuhashi25, Koushiro Ohtsubo26, Kentaro Yamazaki27, Akihito Tsuji28, Wataru Okamoto2,29, Katsuya Tsuchihara2,30, Takeharu Yamanaka31, Izumi Miki2, Yasutoshi Sakamoto2, Hiroko Ichiki2, Masayuki Hata2, Riu Yamashita30, Atsushi Ohtsu1, Justin I. Odegaard32, Takayuki Yoshino1*
- Affiliations
-
- Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- Translational Research Support Section, National Cancer Center Hospital East, Kashiwa, Japan
- Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan
- Department of Gastrointestinal Oncology, National Cancer Center Hospital, Tokyo, Japan
- Biobank Translational Research Support Section, National Cancer Center Hospital, Tokyo, Japan
- Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan
- Department of Gastrointestinal and Medical Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
- Department of Cancer Center, Hokkaido University Hospital, Sapporo, Japan
- Department of Gastroenterology, Saitama Cancer Center, Kitaadachi-gun, Japan
- Department of Gastroenterology, Hepatobiliary and Pancreatic Medical Oncology Division, Kanagawa Cancer Center, Yokohama, Japan
- Department of Colorectal Surgery, Kansai Rosai Hospital, Amagasaki, Japan
- Department of Gastrointestinal Medical Oncology, National Hospital Organization Shikoku Cancer Center, Matsuyama, Japan
- Department of Surgery, National Hospital Organization Osaka National Hospital, Osaka, Japan
- Department of Gastroenterology and Hepatology, University of Tsukuba Hospital, Tsukuba, Japan
- Department of Medical Oncology, Kyorin University Hospital, Mitaka, Japan
- Division of Gastroenterology, Chiba Cancer Center, Chiba, Japan
- Department of Medical Oncology, Kindai University Hospital, Osakasayama, Japan
- Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Medical Oncology, St. Marianna University School of Medicine, Kawasaki, Japan
- Department of Frontier Science for Cancer and Chemotherapy, Graduate School of Medicine, Osaka University, Suita, Japan
- Department of Gastroenterological Chemotherapy, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
- Department of Medical Oncology, Kobe City Medical Center General Hospital, Kobe, Japan
- Cancer Chemotherapy Center, Osaka Medical College Hospital, Takatsuki, Japan
- Department of Surgical Oncology, Graduate School of Medicine, Gifu University, Gifu, Japan
- Division of Medical Oncology, Cancer Research Institute, Kanazawa University, Kanazawa, Japan
- Division of Gastrointestinal Oncology, Shizuoka Cancer Center, Shunto-gun, Japan
- Department of Clinical Oncology, Kagawa University Hospital, Kita-gun, Japan
- Cancer Treatment Center, Hiroshima University Hospital, Hiroshima, Japan
- Division of Translational Informatics, Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, Kashiwa, Japan
- Division of Biostatistics, National Cancer Center Hospital East, Kashiwa, Japan
- Guardant Health, Redwood City, CA
- These authors contributed equally: Yoshiaki Nakamura and Hiroya Taniguchi
- DOI
- 10.1038/s41591-020-1063-5
- Data of publication
- October 5, 2020
Research funding
This study was conducted with the following support:
- Funding from companies participating in SCRUM-Japan
- Practical Research for Innovative Cancer Control, Japan Agency for Medical Research and Development (AMED)
“Creation of Comprehensive Catalog on Clonal Evolution of Cancer Genome Alteration in Metastatic Colorectal Cancer Utilizing Circulating Tumor DNA Analysis”(Principal investigator: Yoshiaki Nakamura) - The National Cancer Center Research and Development Fund (31-A-5) (Principal investigator: Atsushi Ohtsu)
Glossary
- *1 Screening test
- In screening tests for clinical trials, patients are tested and interviewed to check whether they meet eligibility requirements. Items include height, weight, body temperature, blood pressure, blood collection, respiratory examination, electro-cardiogram, CT scan and interview.
- *2 Cancer precision medicine
- Medical treatments adapting to constitutions and medical conditions of individuals by identifying cancer-associated gene alterations by simultaneous testing of multiple genes.
- *3 SCRUM-Japan (Cancer Genome Screening Project for Individualized Medicine in Japan)
- An industry-academia collaborative cancer genome screening project integrated both LC-SCRUM-Japan (currently LC-SCRUM-Asia) for patients with lung cancer started in 2013 and GI-SCREEN-Japan (currently MONSTAR-SCREEN) for patients with gastro-intestinal cancer started in 2014. The project aims at screening genomic alterations in patients with solid tumors, over 10,000 patients with advanced solid cancer have participated in this study since its launch in February 2015. Outcomes of this study include regulatory approvals of 8 new drugs and 9 in vitro diagnostics. With participation of over 200 medical institutions and 17 pharmaceutical and diagnostics companies, academia, hospitals and industries are developing drugs and diagnostics together that match genetic alterations of cancer patients in Japan.
References (in Japanese):- National Cancer Center Japan | SCRUM-Japan
- National Cancer Center Japan | Press release “The launch of the third period of a nation-wide industry-academia-government collaboration project SCRUM-Japan”
- National Cancer Center Japan | Press release “Analysis of 73 genetic alterations with blood in SCRUM-Japan GI-SCREEN – moving towards the achievement of personalized medicine using liquid biopsy”
- *4 Profiling
- An inspection to check multiple genes in one assay using a tumor tissue sample.
- *5 Driver genes
- Genes that play direct and important roles in carcinogenesis and cancer development, such as oncogenes and tumor suppressor genes.
Contact information
For media inquiries
National Cancer Center Japan
Office of Public Relations, Strategic Planning Bureau (Kashiwa Campus)
6-5-1 Kashiwanoha, Kashiwa-shi Chiba, 277-8577 Japan
Telephone: +81-4-7133-1111
FAX:+81-4-7130-0195
E-mail:ncc-admin(at)ncc.go.jp
Contact information for AMED projects
Practical Research for Innovative Cancer Control
Division of Basic Medical Research
Department of Basic Medical Research
Japan Agency for Medical Research and Development (AMED)
22F Yomiuri Shimbun Bldg. 1-7-1 Otemachi, Chiyoda-ku, Tokyo 100-0004 Japan
Telephone:+81-3-6870-2286
E-mail:cancer(at)amed.go.jp
* Replace "(at)" with "@".
10/06/20
Last updated 10/06/20

