Completed Project Focused on Developing Key Evaluation Technology: Manufacturing Technology for Industrialization in the Field of Regenerative Medicine(termination)
Outline
| Key Fields | Japan Regenerative Medicine Project(1st) |
|---|---|
| R&D phase | Clinical Trials, None |
| Contact |
|
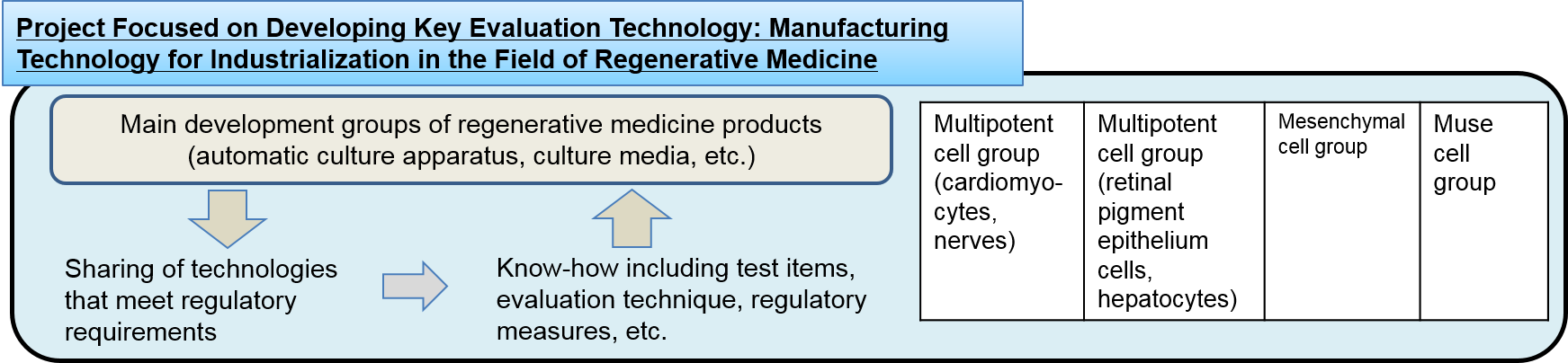
By developing technologies for culturing the currently unestablished iPS and other stem cells in high quality and quantity, and promoting standardization of the technologies, the project aims to accelerate industrial applications of iPS and other stem cells in the field of regenerative medicine and enhance the global competitiveness of Japanese companies in the markets for cell culture apparatus and other peripheral products imperative for developing regenerative medicine products. Specifically, based on process management technologies indispensable for securing a range of processes, including cell expansion, induction of differentiation, quality control, processing, and preservation (freezing, thawing), and for ensuring the accuracy and certainty of the processes, automation instruments for each process and peripheral products including culture media and substrates are being developed. The processes will be required for manufacturing regenerative medicine products for applicable diseases and operation methods as well as manufacturing and processing human stem cells that serve as their raw material.
Last updated 04/19/17

