Completed Department of Clinical Research and Trials
Overview of Department of Clinical Research and Trials
The Department of Clinical Research and Trials consists of the Division of Clinical Research and Trials and the Office of Regulatory Science and Clinical Research Support.
In order to establish a system that facilitates consistent practical application of the results of university-based innovative basic research, the Division of Clinical Research and Trials accelerates integration of the Translational Research Core Centers and the Core Clinical Research Hospitals, and supports development of seeds for drugs and medical devices by enhancing the functions of the hub centers, including the securing and cultivation of human resources and the strengthening of networks covering the organizations outside the hub centers. Moreover, the division promotes enhancement of the functions of academic research organizations (ARO) and the establishment of a system that enables the implementation of high-quality clinical research and trials pursuant to ICH-GCP.
The Office of Regulatory Science and Clinical Research Support secures and fosters human resources, including clinical research coordinators, and promotes “research that serves the need for appropriate and prompt science-based prediction, evaluation and judgment of the quality, efficacy and safety of innovative drugs, medical devices and products for regenerative medicine" (regulatory science research) so that the practical application of such can be promoted quickly and safely. By taking these approaches, the office contributes to the development and standardization of technologies for evaluation of the quality, efficacy and safety of such products, the explicit definition of judgment criteria, and the clarification of scientific requirements for approval review.
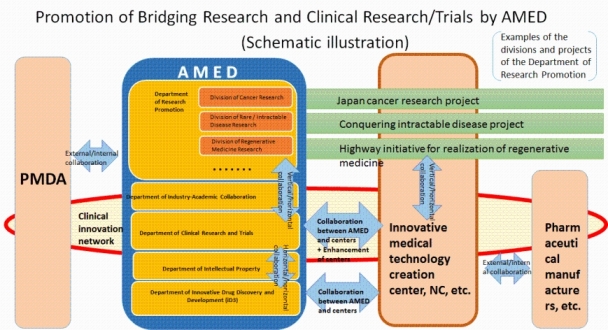
Now that the Japan Agency for Medical Research and Development (AMED) has been established, the department will fortify the hub centers such as the Core Clinical Research Hospitals and National Centers (NCs) and promote efficient innovation through exploring seeds outside the hub centers, and clearly dividing functions between hub centers and between hub and non-hub centers in order to conduct, mediate and support a variety of research activities regarding promising seeds ranging from translational research to investigator-led clinical trials in collaboration with the Department of Innovative Drug Discovery and Development, Department of Research Promotion, Department of Industrial-Academic Collaboration and Department of Intellectual Property, which were incorporated into AMED, and, moreover, in close collaboration with the Pharmaceuticals and Medical Devices Agency (PMDA).
Thus, the direction of the actions to be taken by AMED (Department of Clinical Research and Trials) are summarized below:
- At first, by reference to the collaboration between the National Institutes of Health (NIH) and National Cancer Institute (NCI) in the U.S., we further fortify the hub centers by strengthening the collaboration between AMED (Department of Clinical Research and Trials) and the hub centers. Then,
- We fortify the collaboration inside and outside AMED by promoting
- “Vertical-horizontal" collaboration (collaboration between key fields, collaboration between hub centers)
- “Horizontal-horizontal" collaboration (collaboration among the Department of Innovative Drug Discovery and Development, Department of Industrial-Academic Collaboration and Department of Intellectual Property)
- “External-internal" collaboration (collaboration with PMDA and industrial sectors)
In this manner, we attempt to create an environment conducive to the efficient promotion of research and development of the innovative and novel seeds discovered in Japan under the explicit exit strategy based on collaboration with PMDA and the all-Japan system led by the hub centers such as the Core Clinical Research Hospitals and their networks.
Division of Clinical Research and Trials
Last updated 10/11/17

