Medical Research and Development Programs Focused on Technology Transfer: Adaptable and Seamless Technology Transfer Program through Target-Driven Research and Development (AMED・A-STEP)
Outline
| Key Fields | Medical Device Development(1st), Other programs(1st) |
|---|---|
| R&D phase | Applied Study, Nonclinical Study/Pre-clinical Study, Clinical Trials |
| Contact |
|
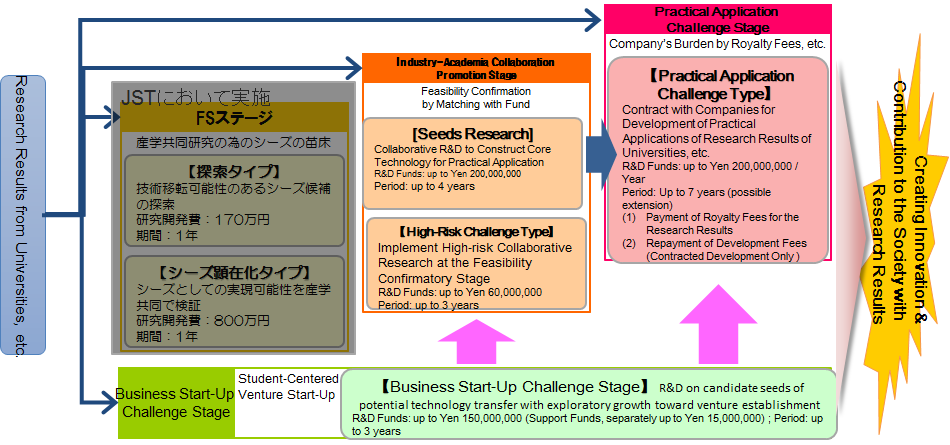
Promoting technology transfer from academia to industry so that the research outputs of universities and public research institutions, which could create significant economic impacts, can be efficiently put into practical application.
This program supports collaborative industry-academia research and development (R&D) based on the results of high-quality basic research (research output, intellectual property, etc.) to ensure that the benefits of such research are passed on to Japanese society. Depending on the R&D phase and the objectives of each particular project, A-STEP determines the optimal R&D funding and R&D period to enable the seamless pursuit of medium- to long-term R&D. Through this approach, the program aims to bridge the gaps between academic research results and industrial needs to realize highly effective and efficient innovation.
This program was transferred from JST to AMED in April 2015, and after that AMED has not received any applications to this program. This program has evolved and is integrated into the ACT-M scheme.
Management and Evaluation Framework
- PO (Program Officer)
-
- Susumu Tanabe, Former General Manager of Terumo Medical Pranex & Former Deputy General Manager of R&D Center, TERUMO CORPORATION
- Yuzuru Matsuda, Ph.D, President, KATO MEMORIAL BIOSCIENCE FOUNDATION
Last updated 07/07/17

