Project Focused on Developing Key Evaluation Technology: Evaluation for Industrialization in the Field of Regenerative Medicine(termination)
Outline
| Key Fields | Japan Regenerative Medicine Project(1st) |
|---|---|
| R&D phase | Clinical Study, Clinical Trials, None |
| Contact |
|
Overview
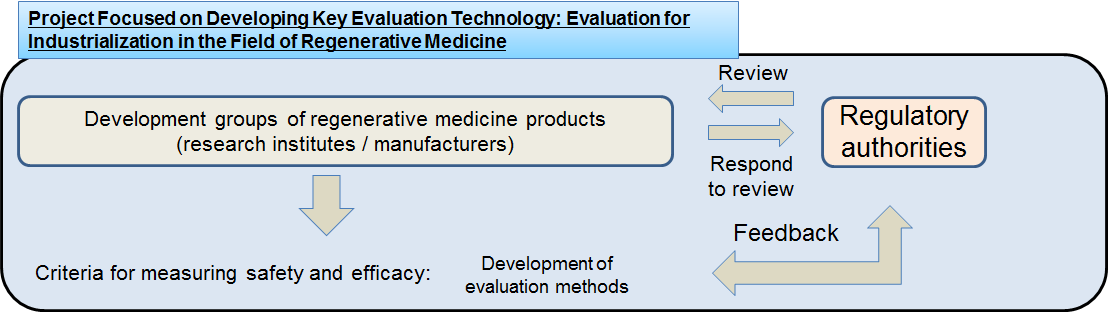
With a view to fostering Japan's supreme technological seeds into products in the field of regenerative medicine, the project aims to develop methods of evaluating safety, efficacy and other criteria, which development companies are required to present upon obtaining approval and undergoing conformity assessment. Specifically, the project will define the evaluation criteria and their indicators for measuring safety and efficacy specific to individual products as well as for measuring equivalence when changes have been made to production/processing processes. In addition, a rational evaluation method will be developed to build a solid foundation for the practical application and industrialization of regenerative medicine products to follow.
Last updated 04/19/17

