Development of Medical Devices through Collaboration between Medicine and Industry
Outline
| Key Fields | Medical Device Development(1st) |
|---|---|
| R&D phase | Basic Study, Applied Study, Nonclinical Study/Pre-clinical Study, Clinical Study, Clinical Trials, Post Marketing, Clinical Research, None |
| Contact |
|
Overview
This program stimulates the medical device industry and advances the quality of medical care in Japan by promoting new entry of small and medium enterprises, venture businesses, and other parties having skills of the medical device industry. It also facilitates collaborations between industry and medical institution for the development and programming of medical devices by taking advantage of the “manufacturing technology” on which Japan prides itself.
Program Overview
FY2018 budget
3.04 billion
Program Outline
The development/commercialization programs (subsidized)
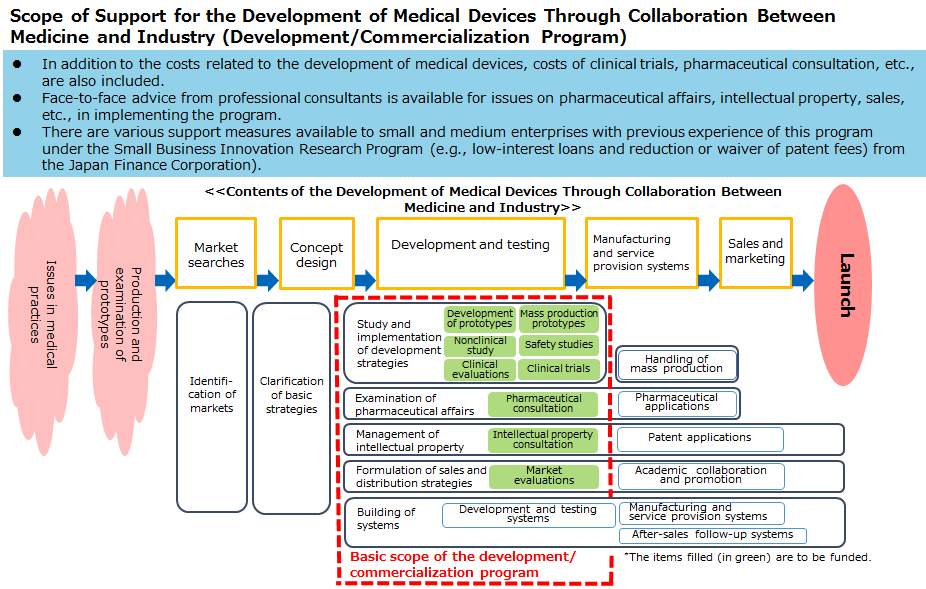
We solicit applications for proposals for the commercialization of medical devices and decide on programs to be selected. The selected consortium will promote the development of devices including developing prototypes and mass production prototypes for commercialization, conducting nonclinical studies, or clinical evaluations. In addition, the consortium will make preparations for commercialization that include strategies for pharmaceutical affairs, intellectual property, sales, and distribution. The consortium will also provide assistance for pharmaceutical affairs, commercialization, intellectual property, technology, etc., mainly through consultation on commercialization.
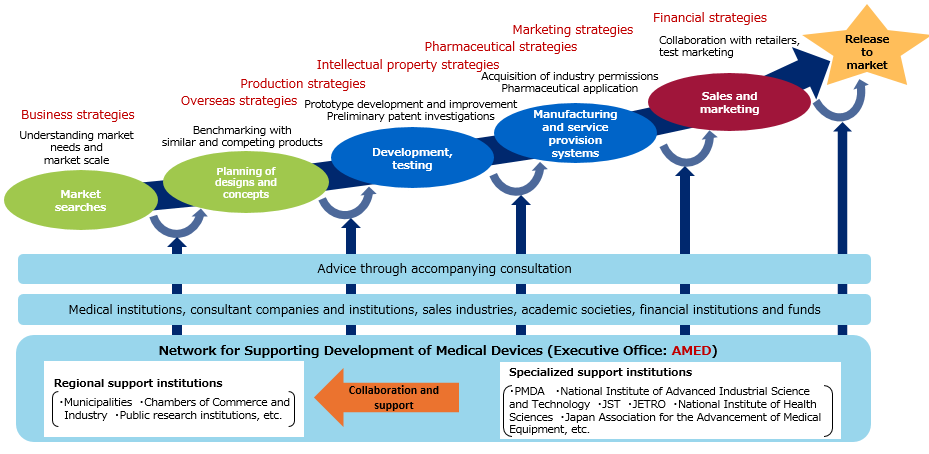
Networks for supporting development of medical devices (contracted)
With contributions to future medical device policies in mind, we promote collaborative networks between the national government and local regions so that medical device developers, including newcomers, can receive various support including pharmaceutical affairs, intellectual property, technology, and marketing in an integrated manner. This work aims at autonomous progress of development/commercialization of medical devices through collaboration between medicine and engineering.
Program Management Framework
PS (Program Supervisor), PO (Program Officer)
- PS
-
- Yoshiyuki Taenaka, M.D., Ph.D., Emeritus Member,National Cerebral and Cardiovascular Center/Specially Appointed Professor, Global Center for Medical Engineering and Informatics, Osaka University.
- PO
-
- Ichiro Sakuma, PhD., Professor, Medical Device Development and Regulation Research Center, School of Engineering, The University of Tokyo.
Organizational framework
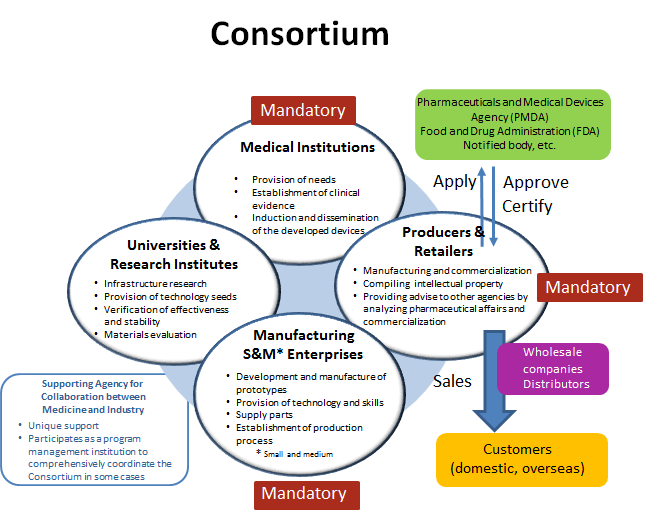
It must be implemented by the consortium consisting of small and medium sized manufacturing enterprises, manufacture and marketing enterprises, and medical institutions.
Subsidized project period
Within 3 years
Basic scope
Last updated 06/21/19

