Public Understanding and Information Sharing on Research Ethics (Ethical, legal and social issues on the practical application of genome medicine)(termination)
Outline
| Key Fields | Other programs(1st) |
|---|---|
| R&D phase | None |
| Contact |
|
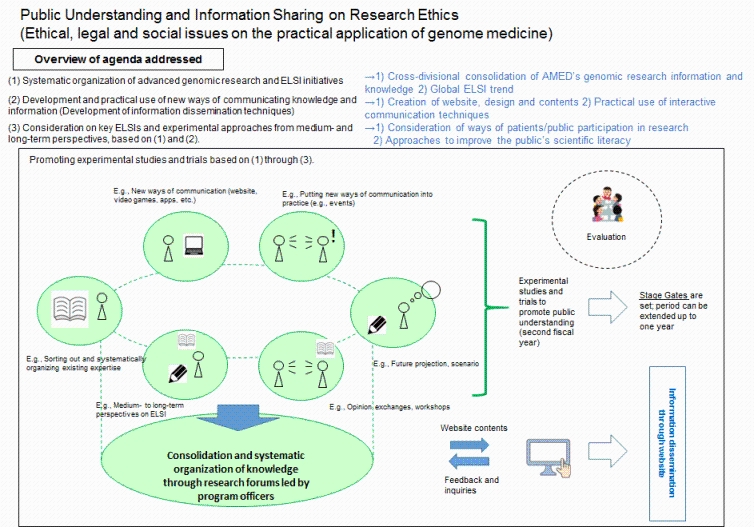
For the realization of genomic medicine, it is needed to promote research participation and communication activities to raise public awareness as strategic public relations measures, as stated in the Interim Report of the Council for the Realization of Genomic Medicine (July 2015; headquarters for the government's healthcare policy). It is also meaningful to share and systematically organize information of ongoing studies on Ethical, Legal and Social Issues (ELSI) implemented under AMED programs and others, and strive to enhance understanding among related parties. Therefore, in fields related to genomic research, this program aims to encourage information sharing and public understanding on research ethics by promoting experimental studies and trials such as creating viewer-friendly websites, sorting out existing expertise, addressing new challenges, and developing and practicing new approaches to communicating knowledge and information.
Management and Evaluation Framework
- PS (Program Supervisor)
-
- Akira Akabayashi, M.D., Ph.D., Professor, Department of Biomedical Ethics, Graduate School of Medicine, The University of Tokyo
- PO (Program Officer)
-
- Takayuki Shiose, Ph.D., Associate Professor, The Kyoto University Museum
- Jusaku Minari, Ph.D., Associate Professor, Uehiro Research Division for iPS Cell Ethics,
Center for iPS Cell Research and Application, Kyoto University
Last updated 07/07/17

