Project for the Certification of Ethical Review Committees(termination)
Outline
| Key Fields | Translational & Clinical Research Core Centers(1st) |
|---|---|
| R&D phase | Clinical Study, Clinical Research, None |
| Contact |
|
Overview of the Program
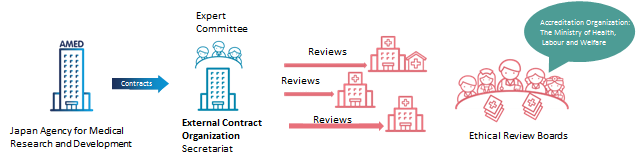
This program conducts document reviews, on-site inspections to certify ethical review committees. It inspects the details of implementation, structure of the review committee members, review statuses, and other relevant issues during the document review and on-site inspections. This program aims to raise the quality of research reviews by certifying ethical review committees that are capable and credible to judge ethical and scientific validities in research conduct.
Last updated 04/06/17

