Project for Development of Central Institutional Review Board
Outline
| Key Fields | Translational & Clinical Research Core Centers(1st) |
|---|---|
| R&D phase | Clinical Trials, Clinical Research, None |
| Contact |
|
Overview
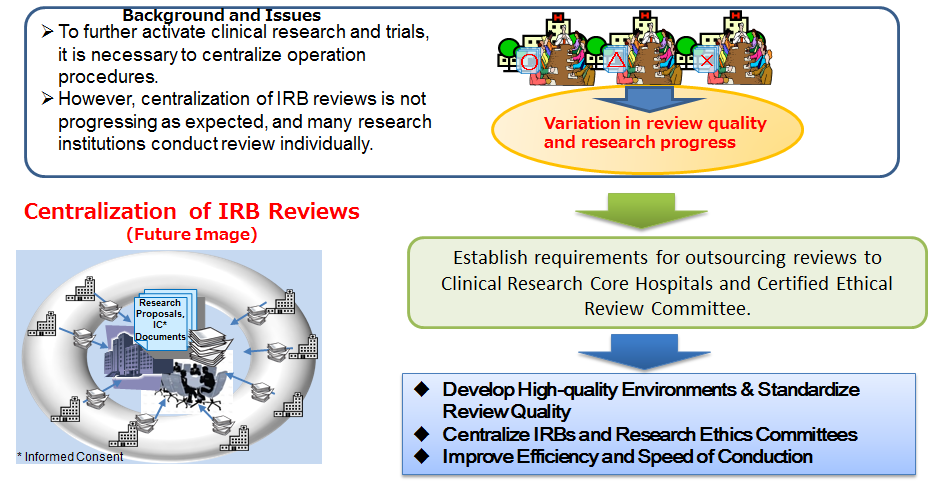
To promote Central Institutional Review Board (IRB), this program establishes requirements to outsource reviews to Clinical Research Core Hospitals and Certified Ethical Review Committees. It is a program to build a model to maintain and operate Central IRB.
The program further aims to standardize review quality in multicenter studies, consolidate IRBs and Ethical Review Committees, and improve efficiency and conduction speed of clinical trials and researches, thereby leading to smooth implementation of Central IRB.
Last updated 05/15/17

