Basic Science and Platform Technology Program for Innovative Biological Medicine(termination)
Outline
| Key Fields | Drug Discovery & Development(1st) |
|---|---|
| R&D phase | Basic Study, Applied Study, Nonclinical Study/Pre-clinical Study, Clinical Study, Clinical Trials, Post Marketing, Clinical Research, None |
| Contact |
|
Overview
Biological Medicines are expected to be highly specific for their targets and have strong levels of efficacy. Japanese pharmaceutical companies trying to develop Biological Medicines face several technical challenges, including technologies to deliver Biological Medicines to their target organs, tissues, cells, or intracellular molecules. Other challenges are know-how to improve their potency and to stabilize nucleic acid medicines. Technology to modify the carbohydrate structure of Biological Medicines is also needed.
The goals of our program are to resolve those technical challenges in developing Biological Medicines by creating innovative technologies, which contributes to strengthening the productivity of Japanese pharmaceutical companies.
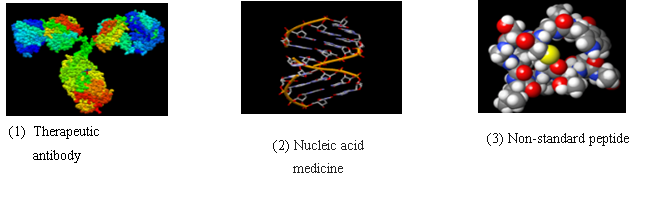
We support the members of our program in developing innovative core technologies related to Biological Medicines, which include non-standard peptides, nucleic acids, and therapeutic antibodies. The created core technologies are being transferred to pharmaceutical companies or related organizations at the end of our program.
Management and Evaluation Framework
- PS (Program Supervisor)
-
- Toshio Miyata, M.D., Ph.D., Professor, Tohoku University
- PO (Program Officer)
-
- Yoshihiro Ohtaki, Ph.D., President, Biofrontier Partners, Inc.
- Tadashi Horiuchi, Ph.D., Guest Professor, Clinical and Translational Research Center, Keio University Hospital, Keio University School of Medicine
Last updated 07/07/17

