Research on Regulatory Science of Pharmaceuticals and Medical Devices
Outline
| Key Fields | Drug Discovery & Development(1st), Medical Device Development(1st) |
|---|---|
| R&D phase | Basic Study, Applied Study, Nonclinical Study/Pre-clinical Study, Clinical Study, Clinical Trials, Post Marketing, Clinical Research, None |
| Contact |
|
Overview
Medical products such as pharmaceuticals, medical devices, cellular and tissue-based products are special industrial products because of the following situations: (1) these products relate to human lives; (2) it is difficult to complete the evaluation of efficacy and safety at the time of approval; and (3) the public insurance system bears part of the cost in Japan. Therefore, a development difficulty is pointed out, especially for innovative medical products, due to the fact that they are approved under tight regulations and strict evaluations, which also continue in post-marketing. To overcome the difficulty, it is important that medical product developers, regulatory personnel, and/or academia conduct regulatory science research on these medical products, and publish fruits of the research as guidelines on its development, etc, or documents (articles, reports, etc.), whose contents have not reached the formation of guideline. It makes it possible to organize the points that tend to conflict between developers and regulatory authorities with transparency by exchanging opinions among stakeholders based on such published documents. As the results, the speed of development would be accelerated and the approval of applications and reviews would be facilitated. Ultimately, it leads to appropriate and early clinical applications and practical applications of new medical technologies.
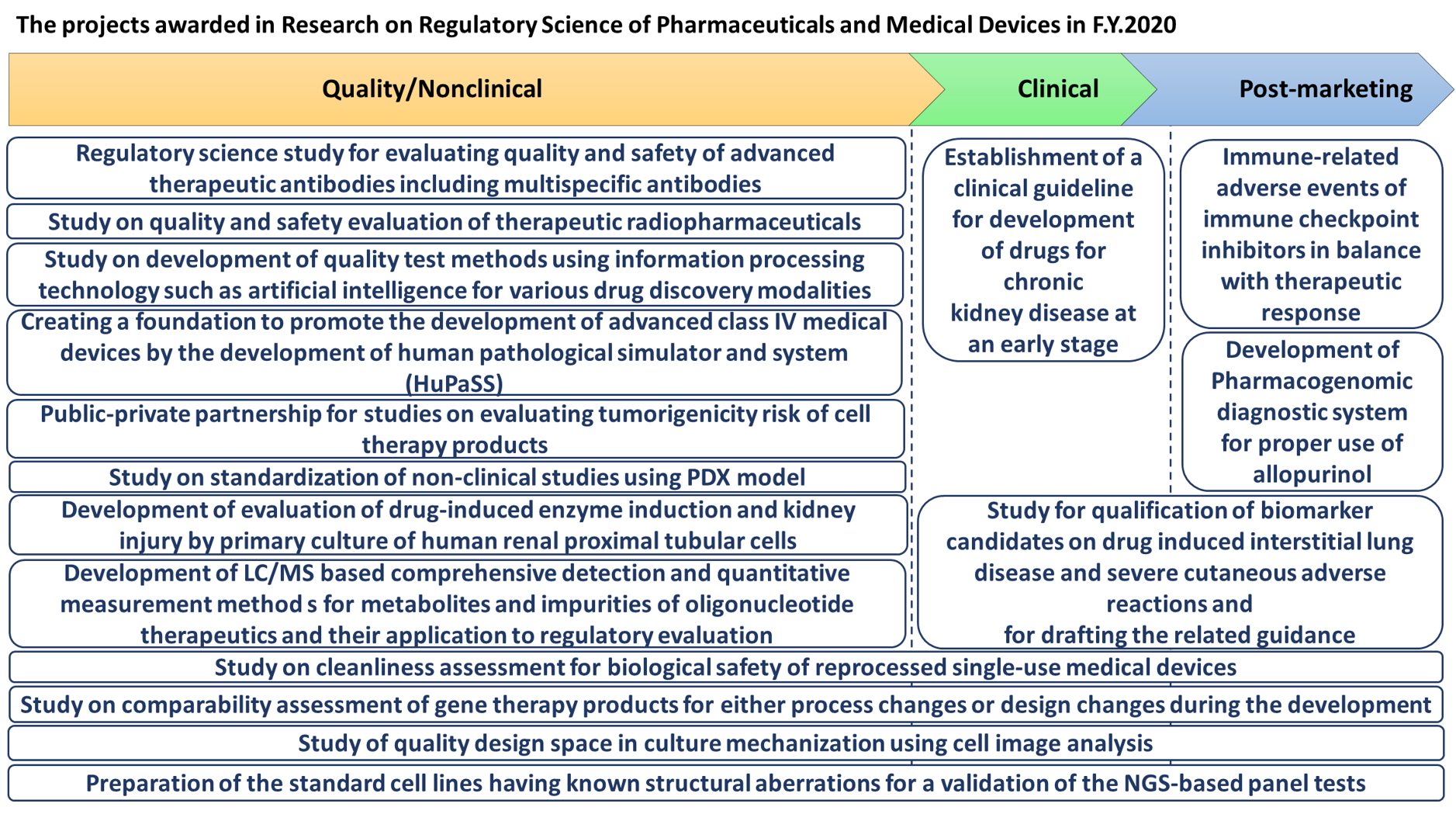
Based on the above background, this program publically recruits research projects focusing on regulatory science research. It aims to the followings; (1) formulation of regulatory guidelines and criteria with scientific rationality and social justification, or (2) implementation of the evaluation methods equipped with the state-of-the art technology, and (3) propose of the international regulatory standards on pharmaceuticals and medical devices etc. all over the world, and so on.
Management and Evaluation Framework
- PS (Program Supervisor)
-
- Haruhiro Okuda, Ph.D., Assistant of President, Pharmaceutical and Medical Device Regulatory Science Society of Japan
- PO (Program Officer)
-
- Takeo Katakura, Ph.D., Visiting Researcher, National Institute of Health Sciences (NIHS)
- Kazuhiro Sase, M.D., Ph.D., Professor, Clinical Pharmacology & Regulatory Science, Graduate School of Medicine, Juntendo University
- Akifumi Matsuyama, M.D., Ph.D., Director, Center for Reverse Translational Research, Osaka Habikino Medical Center
- Takao Yamori, Ph.D., Professor, Teikyo Academic Research Center
Last updated 09/15/20

