Division of Health and Clinical Data Program for an Integrated Database of Clinical and Genomic Information
Outline
| Key Fields | Japan Genomic Medicine Program(1st) |
|---|---|
| R&D phase | None |
| Contact |
|
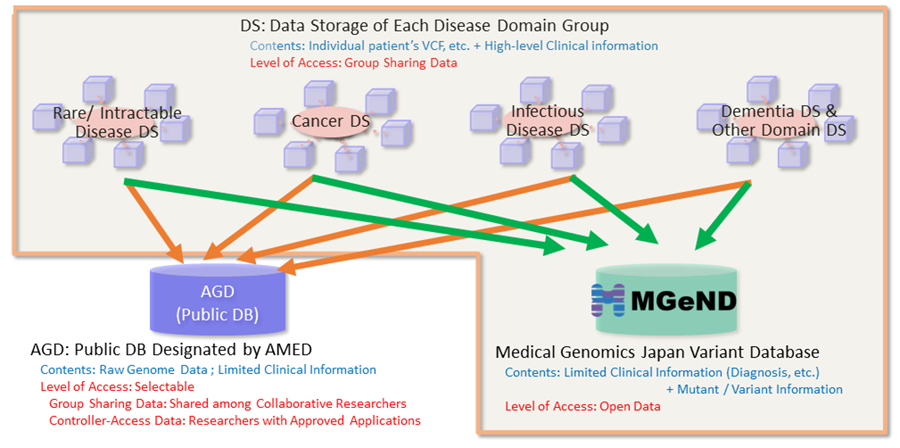
The objective of this program is to improve the infrastructure for sharing clinical and genomic information in order to accelerate R&D, and to promote genomic medicine in a clinical setting.
In this program, we promote studies that clarify the relationship between genomic information and clinical characteristics such as information about diagnosis and treatment for Japanese patients in the fields of rare/intractable diseases, cancers, infectious diseases, dementia, and other diseases. Furthermore, it develops data storage (DS) for each disease group and integrated databases (IDB), which gather clinical and genomic integrated information to be utilized for clinical and research purposes.
Management and Evaluation Framework
- PS (Program Supervisor)
-
- SHINICH Kohsaka, M.D., Ph.D., Director-General Emeritus, National Institute of Neuroscience, national Center of Neurology and Psychiatry
- PO (Program Officer)
-
- NAKAGAWA Hidewaki , M.D., Ph.D., Team Leader, Laboratory for Genome Sequencing Analysis, RIKEN Center for Integrative Medical Sciences
- SUGANO Sumio, M.D., Ph.D., Lecturer, Molecular Epidemiology, Medical Research Institute, Tokyo Medical and Dental University
Last updated 02/16/23

