Division of Technology Transfer Acceleration Transformative Research for Medical Innovation
Outline
| Key Fields | Other programs(1st) |
|---|---|
| R&D phase | Applied Study, Nonclinical Study/Pre-clinical Study |
| Contact |
|
Overview
This program supports industry-academia collaborative research and development (R&D) with "proposal public offerings" so that collaboration between universities, businesses, hospitals, etc., will be developed and "technology seeds" derived from academia can be smoothly and effectively transferred to (put to practical use in) industry (businesses). In the program, the set-up scheme (Acceleration Transformative Research for Medical Innovation Set-up Scheme [ACT-MS]), in particular, aims to strategically transfer "challenging technology seeds in early stages" owned by universities and other related organizations to businesses working to apply them to healthcare. The system intensively supports R&D that can clarify and solve issues related to the seeds of technology.
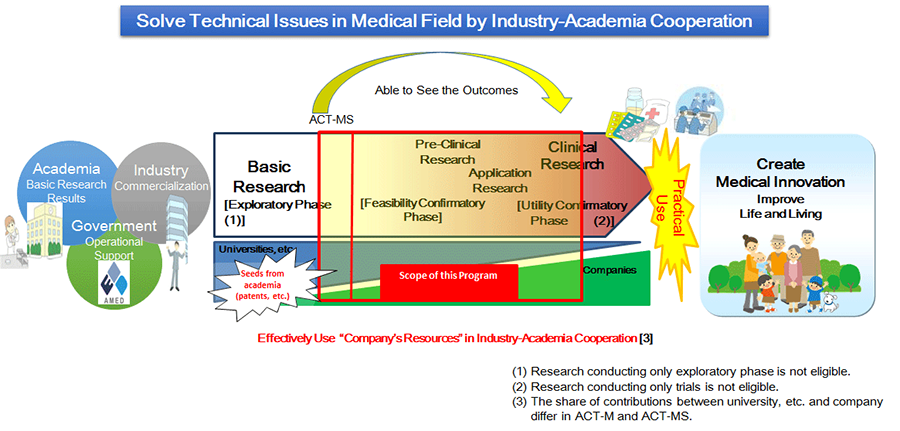
- Research conducted only for the exploratory phase is excluded.
- Research conducted only for clinical trials is excluded.
- The share proportion between universities and other related organizations and businesses will depend on whether they are under the Acceleration Transformative Research for Medical Innovation (ACT-M) or ACT-MS.
Program Overview
FY2018 budget
1.3 billion yen
Target areas
Projects on the three kinds of "Life" (biosis, livelihood, and human life) are targeted.
- Biosis
- Perspectives to link results of academic research on biological phenomena to "new healthcare."
- Livelihood
- Perspectives to improve quality of life (QOL) associated with healthcare. This includes not only research to pursue the sophistication of modern standard medical care that has been developed so far, but also studies that attempt to scientifically verify or construct a logical foundation for "alternative medicine" or "integrative medicine" that can potentially surpass current healthcare in the future.
- Human life
- Long-term perspectives, including preventive or preemptive treatment from youth to old age. This includes research that utilizes genetic information (e.g., acquired changes).
R&D themes
There are two R&D themes in this program as shown below:
| Theme title | Overview |
|---|---|
|
This program calls for R&D aiming to achieve the effective and efficient development of the “buds" of medicines as manifested in academia at companies, etc., and transfer new peripheral technology supporting drug manufacturing to companies. |
|
This program calls for applications for R&D that comprehensively perceives diseases and aims to create innovative medical technologies and devices on important diseases to be addressed under collaborative R&D with companies regarding the academia's seed-stage resources based on the new knowledge. |
Composition of the program
This program is composed of two schemes: the ACT-M and the ACT-MS as shown below.
| ACT-MS | ACT-M | |
|---|---|---|
| Purpose | Achieve the breakthrough points for application to healthcare with regard to "challenging technology seeds in early stages." | Solve issues for practical use (commercialization) with regard to "technology seeds that completed the exploratory stage." |
| Contents | With regard to the technology seeds owned by universities and other related organizations, industrial-academic collaboration clarify issues so as to determine the possible applications to healthcare. Furthermore, universities and other related organizations conduct studies (brush-up research) to establish techniques (methods) to solve issues. | With regard to the technology seeds that businesses aim at practical use, industrial-academic collaboration carry out R&D with goal of establishing proof of concept (POC) targeting humans (“verification of possibilities/reproducibility” and “demonstration of practicality” to link to clinical evaluation). |
| Form of proposal | A "joint proposal" by the university and other related organizations that own technology seeds and businesses or entrepreneurships that wish to utilize them (hereinafter referred to as a "set-up business") | A "joint proposal" in which a R&D system enabling clinical use and verification has been constructed by the university or other related organization that owns technology seeds and businesses aiming at its practical use. |
| Form of contract | A direct contract between each participating institution (universities and other related organizations only) and AMED (1-year contract) | A direct contract between each participating institution and AMED (1-year contract) |
| Role of businesses | Share common understandings with universities and other related organizations and clarify the breakthrough points for technology seeds; then draw up and implement plans to put them to practical use and commercialization (business models) after achieving the goals. | After defining the roles of the universities and related organizations, conducts their own R&D as well. Although it is not a matching fund, specify and present the R&D costs, personnel expenses, etc., that are planned to be paid by businesses. |
| R&D period | Within 2 years in principle (approx. 1 year and 6 months) (FY2018 to FY2019) |
Within 3 years in principle (approx. 2 years and 6 months) (FY2018 to FY2020) |
| R&D system | Industry-academia collaborative R&D team The project leader (the representative of the application) should be a researcher at a university or other related organization. |
Industry-academia collaborative R&D team The project leader (the representative of the application) may be either from a university or other related organization or business. |
| Who is eligible to receive assistance for R&D costs | Universities and other related organizations only | Universities, other related organizations, and businesses |
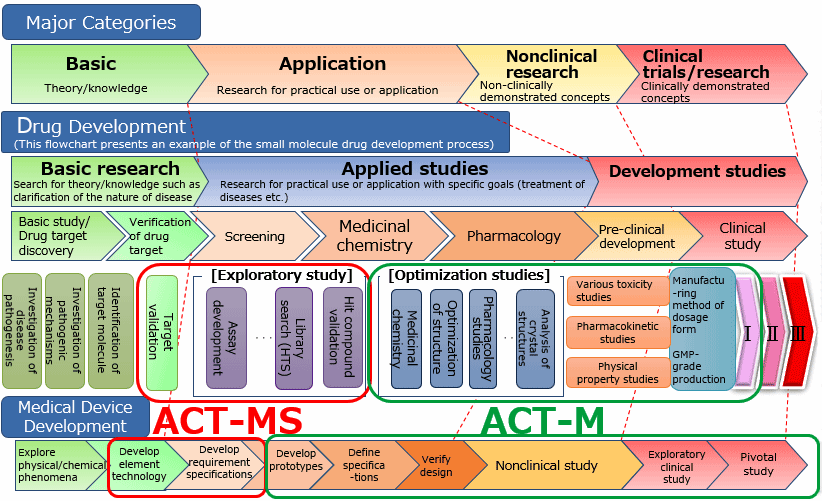
Cases of R&D processes for the development of drugs and medical devices and examples of ACT-M and ACT-MS schemes are illustrated below.
Management and Evaluation Framework
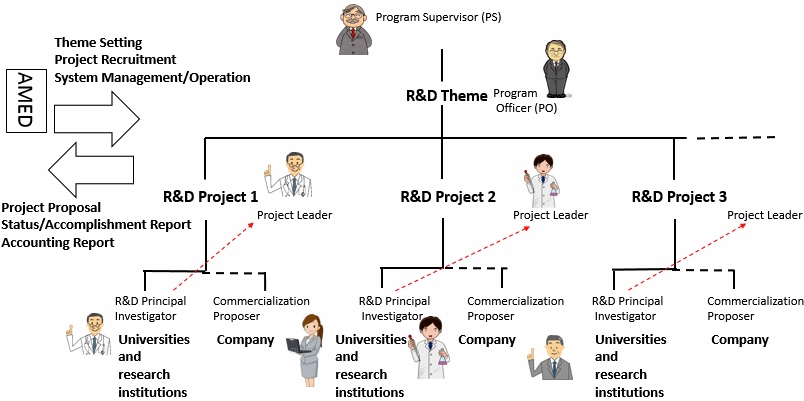
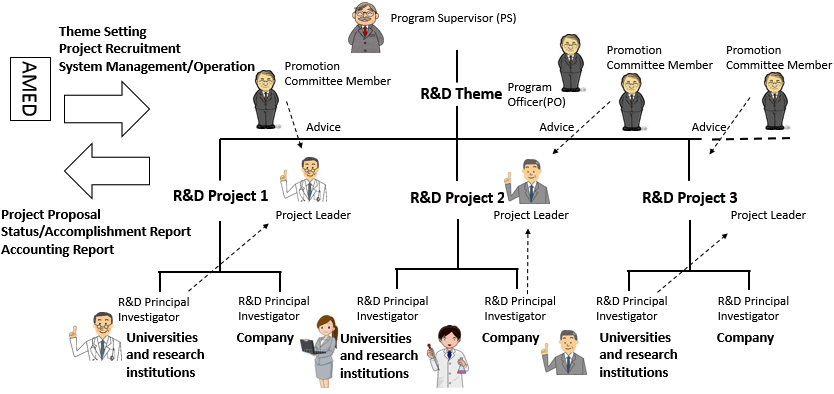
In this program, a program officer (PO) is assigned for each theme under a program supervisor (PS) in the Medical Research and Development Programs Focused on Technology Transfer in order to ensure efficient use of competitive funds and smooth operations that lead to excellent accomplishments.
PS (Program Supervisor)
- Tsutomu Chiba, M.D., Ph.D., Director, Kansai electric power hospital/Professor Emeritus, Kyoto University/Visiting Professor, Kobe University.
PO (Program Officer)
- Theme 1
-
- Seiichi Tanida, Ph.D., Advisor, Kyoto Lifetech Innovation Support Center Industry-Academia-Government Collaboration Business Division Advanced Science, Technology & Management Research Institute of KYOTO
- Theme 2
-
- Kazuhiko Yamamoto, M.D., Ph.D., Deputy Director, RIKEN Center for Integrative Medical Sciences
The PSs and POs understand the progress of the overall program and provide the guidance and advice necessary to facilitate the program. Research institutions and researchers are required to cooperate with the PSs and POs. Reassessment of plans or suspension of R&D project may be requested as needed based on the guidance or advice by PSs and POs.
In addition, promotional committee members are appointed for each R&D project of ACT-M among the project evaluation committee members and support promotion of R&D in collaboration with R&D institutions.
The roles and methods of selection of responsible personnel
| Role | ACT-MS | ACT-M | |
|---|---|---|---|
| The Project Leader | Manages the overall project | Selected from R&D principal investigators | |
| R&D Principal Investigator* | Manages the project at each R&D institution | Selected from the R&D institution of each university or other related institution | Selected from each R&D institution |
| Co-investigator* | Conducts research under R&D Principal Investigator at each R&D institution as needed | Appointed at the R&D institution of each university other related institution | Appointed at each R&D institution |
| Commercialization Proposer (ACT-MS only) |
Develops commercialization plans of the project and shapes R&D | Those who proposes the project at the business (set-up business) | ― |
*It is desirable for a clinician to join as R&D principal investigator or co-investigator.
Acceleration Transformative Research for Medical Innovation Set-up scheme "ACT-MS"
Acceleration Transformative Research for Medical Innovation "ACT-M"
Last updated 06/21/19

