Division of Technology Transfer Strengthening Program for Pharmaceutical Startup Ecosystem
Outline
| Key Fields | Medical Innovation Ecosystem, Cancer, Intractable and rare diseases, Child health and development, Aging and lifestyle related diseases, Mental and neurological disorders and dementia, Infectious disease, other |
|---|---|
| R&D phase | Nonclinical Study/Pre-clinical Study, Clinical Study, Clinical Trials |
| Contact |
|
Overview
Most new drugs in recent years have been developed by pharmaceutical startups, and it is pharmaceutical startups that have succeeded in the development of vaccines early in the current pandemic. Although a large amount of money is required for the development of new drugs, it is difficult to secure the necessary development funds smoothly in Japan's pharmaceutical startup ecosystem compared to Europe and the United States.
In response to this situation, under the "Strategy for Strengthening Vaccine Development and Production Systems" approved by the Cabinet in June 2021, this Program was established to support pharmaceutical startup companies engaged in the commercialization and development of technologies related to vaccines and therapeutics for infectious diseases. Furthermore, in October 2022, the "Priority Issues in a Comprehensive Economic Package regarding the Implementation of the "Grand Design and Action Plan for a New Form of Capitalism" included the following statement regarding this Program: "Going forward, it will enhance support for drug discovery startups by expanding support eligibility to drug discovery fields other than infectious diseases and which face difficult in raising funds".
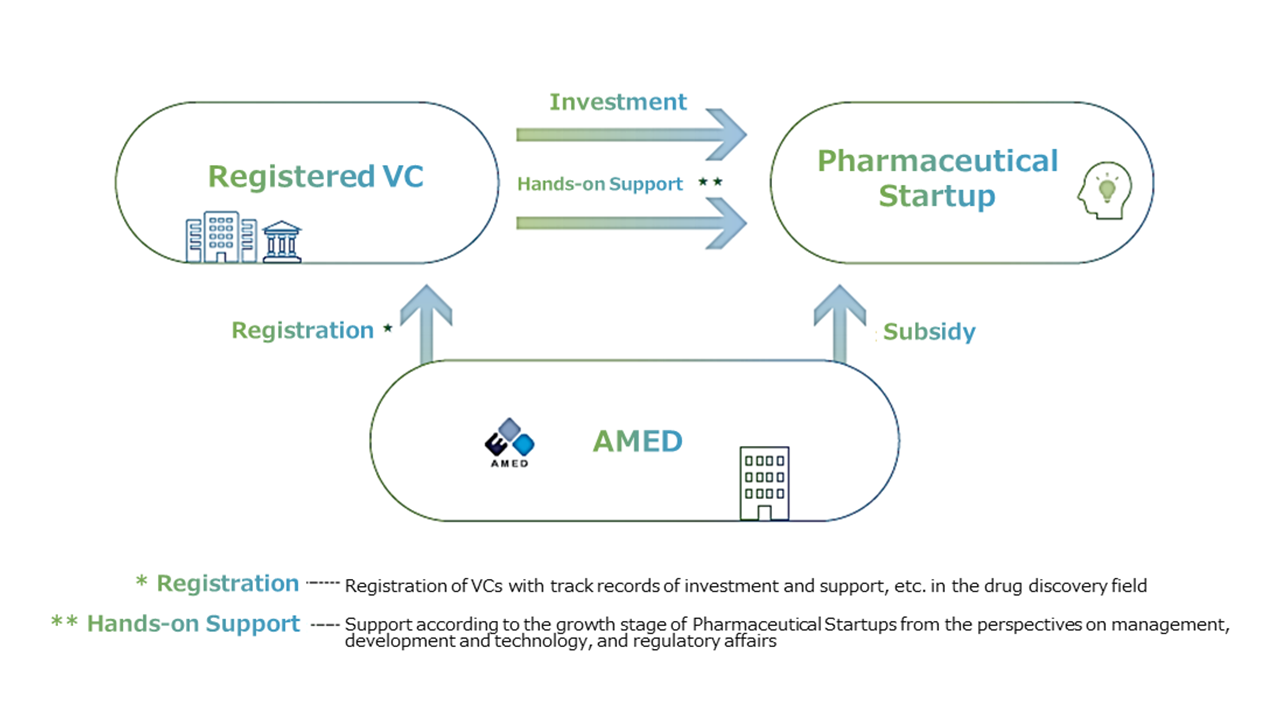
In order to resolve the shortage of sources of development funds on a large scale, this Program registers venture capitals (VCs) that provide hands-on commercialization support specializing in drug discovery, and supports the development and commercialization carried out by Pharmaceutical Startups in the development stage of non-clinical, phase 1, phase 2, or exploratory clinical trials, with the requirement of investment by the registered VCs (Hereinafter referred to as "Registered VC".), thereby raising the foundation of Japan's pharmaceutical startup ecosystem. In particular, we will actively support commercialization plans in overseas markets in addition to Japan in order to achieve sufficient sales and growth.
Pharmaceutical startups, which are Japanese subsidiaries of foreign corporations established for fund-raising or commercialization in overseas markets, will also be eligible for support.In this Program, AMED subsidizes the practical development of pharmaceuticals conducted by Pharmaceutical Startups in which registered VCs invest more than 1/3 of total expenses covered by the Subsidy.
PROGRAM SCHEME
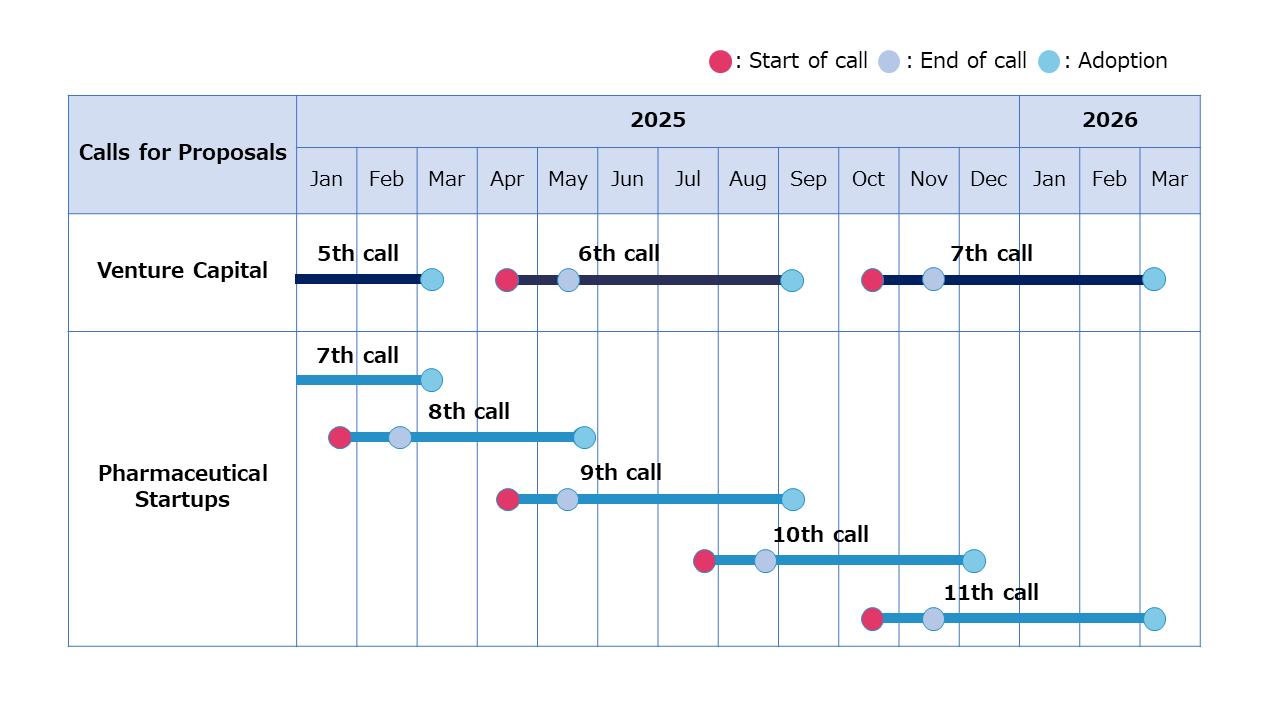
Future Calls for Proposals are scheduled as shown in the table below (subject to change depending on circumstances).
Evaluation and Management System
In order to ensure efficient utilization of competitive research funds and smooth implementation to produce excellent results, a program director (hereinafter referred to as "PD".) is assigned to each integrated project, and a program supervisor (hereinafter referred to as "PS".) and a program officer (hereinafter referred to as"PO".) are assigned to each program.
PS, PO, etc. will grasp the progress of the entire Program and provide necessary guidance and advice for the smooth promotion of the Program. In addition, research institutions and researchers are obliged to cooperate with PS, PO, etc. Based on guidance and advice provided by PS, PO, etc., the plan of supporting Subsidized Projects may be reviewed or suspended (including early termination due to the achievement of the plan.) as necessary.
PS
INAGAKI Osamu
Former secretary of the Pharmaceutical Evaluation Committee of the Japan Pharmaceutical Manufacturers Association
PO
HASHIMOTO Chika
President Galasus, LLC
HAYASHI Yoshiharu
Chairman SENSHIN Medical Research Foundation Ph.D
WADA Michihiko
Chief Regulatory and Development Officer Newton Biocapital Partners MD & PhD
Registered Venture Capitals
List of Registered VCs (updated October 1, 2025)
| Corporate Name | |
|---|---|
| 4BIO Partners LLP | JAFCO Group Co., Ltd. |
| ANRI Co., Ltd. | JIC Venture Growth Investments Co., Ltd. |
| ANV Management, LLC | Keio Innovation Initiative, Inc. |
| Astellas Venture Management LLC | Kyoto University Innovation Capital Co., Ltd. |
| Beyond Next Ventures Inc. | Mitsubishi UFJ Capital Co., Ltd. |
| Blackstone Life Sciences Advisors LLC | Miyako Capital Inc. |
| Catalys Pacific, LLC | MP Healthcare Venture Management, Inc. |
| Curie Bio Operations, LLC | Newton Biocapital Partners |
| D3LLC | OSAKA University Venture Capital Co., Ltd. |
| DBJ Capital Co., Ltd. | Remiges Ventures, Inc. |
| DCI Partners Co., Ltd. | Saisei Ventures LLC |
| Eight Roads Ventures Japan(Eight Roads Capital Advisors Hong Kong Limited) | Taiho Innovations, LLC |
| Eisai Innovation, Inc. | Taiho Ventures, LLC |
| EQT Life Sciences Group B.V. | The University of Tokyo Edge Capital Partners Co., Ltd. |
| F-Prime Capital Partners(Impresa Management LLC) | UTokyo Innovation Platform Co., Ltd. |
| Fast Track Initiative, Inc. | |
Brochure
Last updated 11/11/25



