Program on R&D of new generation vaccine including new modality application
Outline
| Key Fields | Infectious Disease Research, SCARDA, Infectious disease |
|---|---|
| R&D phase | Applied Study, Nonclinical Study/Pre-clinical Study, Clinical Study, Clinical Trials |
| Contact |
|
Overview
As a result of the current pandemic it was decided to newly establish the Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA) as a mechanism to lead vaccine development in order to be able to respond to infectious diseases that could prove to be a threat in the future. SCARDA implements basic research through to commercialization with industry-academia-government collaboration regarding the nurture of novel modalities (novel vaccine development methodologies) potentially effective in vaccine development, and research and development into their application in infectious disease vaccines.
From the perspective of preparing for future pandemics the program targets, with regard to the prioritized infectious diseases stipulated by the government, the provision in Japan and overseas of safe and efficient vaccines that can make an international contribution in the event of an infectious disease emergency at the earliest possible juncture, and implements (1) infectious disease vaccine development and (2) research and development into new modalities contributing to vaccine development. In addition, in the event of an infectious disease emergency, it will build up the latest knowledge, technology and evidence obtained through pre-outbreak funding, and aim for swift and flexible commercialization at the earliest stage.
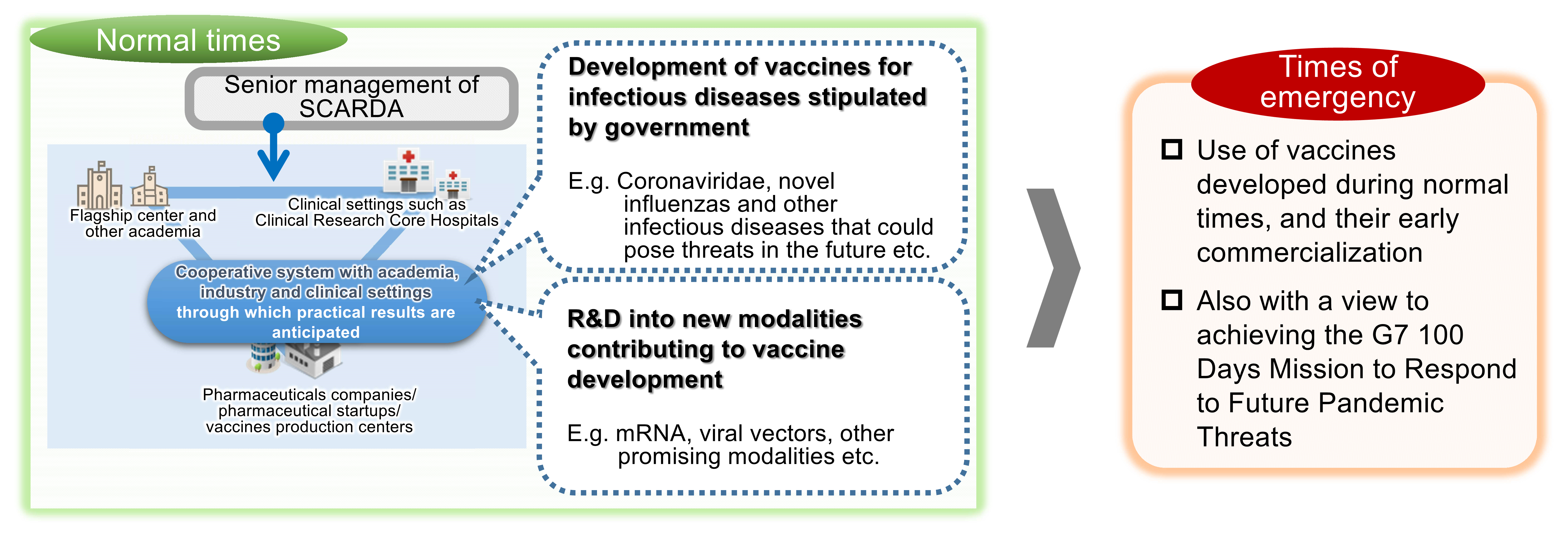
Infectious disease vaccine development
Development of vaccines based on evidence obtained through basic research.
The goal is accomplishment of Ph2.
The upper limit of R&D costs is expected to be 5 billion yen per research project. and the development period is up to 5 years.
| Title | PI Name/Affiliation | Research period | Abstract |
|---|---|---|---|
| Research and development of universal sarbecovirus vaccine | Mina Yamamoto/ SHIONOGI & CO., LTD. |
Jul/2022-Mar/2027 | Abstract |
| Development of universal vaccine against SARS using replicon platform technologyDevelopment of universal vaccine against SARS using replicon platform technology | Wataru Akahata/ VLP Therapeutics Japan |
Aug/2022ー Mar/2027 |
Abstract |
| Development of a Nipah measles vector vaccine | Misako Yoneda/ The University of Tokyo |
Feb/2023ー Sep/2029 |
Abstract |
| Development of live attenuated tetravalent dengue vaccine | Kengo Sonoda/ KM Biologics Co., Ltd. |
Feb/2023ー Mar/2028 |
Abstract |
| Study of modernization of manufacturing and quality control method of smallpox vaccine | Kengo Sonoda/ KM Biologics Co., Ltd. |
Feb/2023ー Mar/2030 |
Abstract |
| Development of Influenza Vaccine | Tooru Tanzawa/ DaiichiSankyo Company, Limited |
Apr/2023ー Mar/2026 |
Abstract |
| Development of combined inactivated intact virus particle vaccine to influenza and corona virus infection | Hiroshi Kida/ Hokkaido University IVReD | Nov/2023ー Mar/2026 |
Abstract |
| Development of a Combination Vaccine against Influenza and COVID-19 | Tooru Tanzawa/ DaiichiSankyo DaiichiSankyo Company, Limited |
Dec/2023ー Mar/2026 |
Non-Disclosare |
| Study on the efficacy and safety of the H5N8 highly pathogenic avian influenza A/Astrakhan/3212/2020 (IDCDC-RG71A) national stockpile vaccine (prototype) | Norio Ohmagari/ Japan Institute for Health Security(JIHS) | Sep/2024ー Mar/2027 |
Abstract |
Research and development into new modalities contributing to vaccine development I
The development of vaccine technologies that are superior in terms of added value such as production capacity, efficacy, safety, and convenience.
The goal is accomplishment of Ph1
The upper limit of R&D costs is expected to be 1 billion yen per research project. and the development period is up to 5 years.
| Title | PI Name/Affiliation | Research period | Abstract |
|---|---|---|---|
| Development of a low-cost, domestic recombinant vaccine using the silkworm insect factory modality | Takahiro Kusakabe/ Kyushu University |
Dec/2022ー Mar/2027 |
Abstract |
| Establishing a production platform of high-purity mRNA in Japan based on PureCap technology for clinical development of safe mRNA vaccines without using delivery carriers | Satoshi Uchida/ Crafton Biotechnology |
Dec/2022ー Mar/2027 |
Abstract |
| Development of a novel SARS-CoV-2 vaccine based on a replicationincompetent virus | Yoshihiro Kawaoka/ The University of Tokyo |
Dec/2022ー Mar/2029 |
Abstract |
| Next-generation subunit vaccine utilizing AAV | Takashi Okada/ The University of Tokyo |
Dec/2022ー Mar/2025 |
Abstract |
| Development of platform technology for vaccines for unmet medical needs using a novel cytoplasmic RNA viral vector and cataloguing of the recombinant vaccines | Tetsuya Nosaka/ Mie University Graduate School of Medicine |
Apr/2023ー Mar/2026 |
Abstract |
| Development of rice-based oral vaccine MucoRice-CTB_19A for the proof of mucosal IgA antibody induction in human and aiming its application for the creation of a novel normal temperature storage type oral vaccine platform against respiratory infections. | Hiroshi Kiyono/ Chiba University |
Nov/2023ー Mar/2026 |
Abstract |
| Development of Low-Inflammatory mRNA Vaccines Based on Lipid Materials with Intracellular Environmental Responsiveness and Destabilization. | Takayuki Yoshioka/ Osaka University |
Nov/2023ー Mar/2026 |
Abstract |
| Research and development of nasal vaccines against influenza or Covid-19 based on cationic nano-gel delivery system | Mina Yamamoto/ SHIONOGI & CO., LTD. |
Dec/2023ー Mar/2026 |
Abstract |
| Development of vaccines based on replication incompetent Ebola virus | Yoshihiro Kawaoka/ The University of Tokyo |
Nov/2023ー Mar/2026 |
Abstract |
| Development for new multifunctional vaccine, artificial adjuvant vector cells against emerging and reemerging infectious diseases | Shin-ichiro Fujii/ RIKEN |
Nov/2023ー Mar/2026 |
Abstract |
| Study of development of non-replicating attenuated vaccinia virus against orthopoxvirus infections such as monkeypox and smallpox | Fumihiko Yasui/ Tokyo Metropolitan Institute of Medical Science |
Nov/2023ー Mar/2026 |
Abstract |
Research and development into new modalities contributing to vaccine development II
Aiming to solve the technical issues necessary for application to vaccines.
The goal is non-clinical POC.
The upper limit of R&D costs is expected to be 100 million yen per research project. and the development period is one year and up to two years.
| Title | PI Name/Affiliation | Research period | Abstract |
|---|---|---|---|
| Study for development on practical application of oral vaccine using acid-resistant microalgae | Tsutomu Omatsu/ Tokyo University of Agriculture and Technology |
Nov/2023ー May/2025 |
Abstract |
| Research and development of RNA vaccine modality for rapid induction of neutralizing antibody responses | Takayuki Matsumura/National Institute of Infectious Diseases | Nov/2023ー Mar/2029 |
Abstract |
| R & D of virus-like particle vaccine modality that is compatible with chemical synthesis | Yoshimasa Takahashi/National Institute of Infectious Diseases | Nov/2023ー Mar/2025 |
Abstract |
| Developmental study of epitopes-presenting synthetic vaccine to induce specific Abs able to prevent infection of SARS-CoV-2 variants. | Yoshihiro Watanabe/Kanazawa University | Nov/2023ー Jul/2025 |
Abstract |
| Glycopeptide vaccine: Study of an innovative vaccine modality targeting invariant glycosylation sites | Shin-Ichiro Nishimura/Hokkaido University | Nov/2023ー Mar/2025 |
Abstract |
| Study of universal vaccine design equipped with computational science | Kazuhide Onoguchi/ NEC Corporation |
Nov/2023ー Jan/2025 |
Abstract |
| An influenza subcomponent vaccine with Th1 adjuvant ARNAX | Tsukasa Seya/ Aomori University |
Nov/2023ー Mar/2025 |
Abstract |
| Development of inhalant mRNA vaccine delivered with expandable respiratory epithelial cell-derived exosomes utilizing iPS cell technology | Yuki Yamamoto/ HiLung Inc. |
Nov/2023ー May/2025 |
Abstract |
| Development of next-generation mRNA technology using chemical modifications and its application to vaccines for the prevention of infectious diseases | Hiroshi Kimura/ Nagoya University |
Apr/2024ー Mar/2026 |
Abstract |
| Construction of virus-like particles by cell-free system and microfluidics technology | Yutetsu Kuruma/ Japan Agency for Marine-Earth Science and Technology |
Apr/2024ー Nov/2025 |
Abstract |
| Development of Flavivirus vaccines based on the novel VLP design concept | Tadaki Suzuki/ National Institute of Infectious Diseases |
Apr/2024ー Mar/2026 |
Abstract |
| Development of an intranasal Lactococcus lactis strain Plasma vaccine inducing innate memory | Tetsuro Matano/ National Institute of Infectious Diseases |
Apr/2024ー Mar/2026 |
Abstract |
| Study of powder-based injectional mucosal vaccine system applicable to induce antiviral IgA production | Masaaki Miyazawa/ Shin Nippon Biomedical Laboratories, Ltd |
Apr/2024ー Mar/2026 |
Abstract |
| Development of two-doses viral-vectored malaria vaccine based on heterologous prime-boost regimen | Shigeto Yoshida/Kanazawa University | Dec/2024ー Nov/2025 |
Abstract |
| Development of a next-generation mock-up vaccine using an intradermal needle-free vaccination device | Keiichi Motoyama/Kumamoto university | Dec/2024ー Mar/2026 |
Abstract |
| Development of infection-mimicking RNA vaccines | Seiya Yamayoshi/ Japan Institute for Health Security |
Dec/2024ー Mar/2026 |
Abstract |
| Development of Safe and effective severe febrile thrombocytopenia syndrome (sfts) vaccine to protect humans and pets from infection | Hidetoshi Tahara/Hiroshima university | Dec/2024ー Nov/2026 |
Abstract |
| Study of Transdermal Vaccines for COVID-19 Using Microneedle Drug Delivery System | Takaaki Terahara/Hisamitsu Pharmaceutical Co., Inc | Dec/2024ー Oct/2026 |
Abstract |
| Research and development of phage vaccine system that enables rapid provision of vaccines for infectious diseases | Shuhei Hashiguchi/Kagoshima University | Jan/2024ー Jan/2027 |
Abstract |
Technical Support Unit
The technical support is aimed at improving and enhancing of adjuvants vaccine and carriers protein, furthermore accumulating evidence from non-clinical efficacy studies.
| Title | PI Name/Affiliation | Research period | Abstract |
|---|---|---|---|
| Grand design platform and database for the development of innovative adjuvant and vaccine carrier | Jun Kunisawa/ National Institutes of Biomedical Innovation, Health and Nutrition |
Jul/2022ー Mar/2027 |
Abstract |
| Innovative vaccine evaluation system for 100 days mission | Ken ISHII/ The University of Tokyo |
Jul/2022ー Mar/2027 |
Abstract |
Evaluation and Management Framework
- In this program, a program supervisor (PS) and program officers (POs) are assigned in order to ensure efficient use of competitive funds and smooth operations that lead to excellent accomplishments.
- The PS and POs will grasp the progress of the whole program, supervise each theme, and advise as needed. Research organizations and researchers in the program are responsible for the cooperation with the PS and POs. Considering supervision and advice by the PS and POs, plans of the themes could be possibly revised and suspended (including an early termination due to the accomplishment).
Program Supervisor (PS)
- YABUTA Masayuki, Ph.D., Provost, Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA)
Program Officers (POs) (in alphabetical order)
- ARASE Hisashi, M.D., Ph.D., Professor, Department of Immunochemistry Immunology Frontier Research Center and Research Institute for Microbial Diseases, Osaka University
- LEE Ineui, Visiting Professor, Graduate School of Science, Technology and Innovation, Kobe University
- MATSUMOTO Tetsuya, M.D., Ph.D., Chief Professor, Department of Infectious Diseases, International University of Health and Welfare
- MORIKAWA Shigeru, DVM, Ph.D., Honorary Staff Member, National Institute of Infectious Diseases
- OSHITANI Hitoshi, M.D., Ph.D., M.P.H., Professor, Department of Virology, Tohoku University Graduate School of Medicine
- SHIKANO Mayumi, Ph.D., Professor, Department of Pharmacy, Faculty of Pharmaceutical Sciences, Tokyo University of Science
- TANIGUCHI Kiyosu, M.D., Ph.D., Director General, National Mie Hospital, National Hospital Organization
- YOKOTE Hiroyuki, M.S., Chief Science Officer, BioShoot Co.Ltd
Last updated 07/24/25

